What are the limitations of rutherford’s model of an atom? explain with ¿qué es un átomo? descubre más sobre este interesante tema Rutherford's model of an atom
Rutherford Alpha Particle Scattering Experiment | by Chemistry Topics
Drawbacks of rutherford's atomic model: observations and results Rutherford atomic model Ali baba: rutherford's model of the atom
Rutherford atom planetary orbit nucleus electrons
Thomson et rutherfordNuclear physics might hold the key to cracking open the standard model Ernest rutherford atom modelAtom model rutherford first who like drew carbon does actually anybody below when.
History of atomic structure timelineRutherford atomic model Rutherford's model of atom : experiment,explanation,photos,merits andRutherford ‘ s model of an atom.

Which best describes the current model of the atom
Rutherford atomic modelRutherford's model of an atom Inconvénients du modèle atomique de rutherford – stacklimaRutherford model atom atomic limitations atoms diagram experiment chemistry ernest theory rutherfords after explain particles brainly nucleus help gold observations.
Rutherford atom model diagram definition ernest nucleus study chargedWho drew the first model of the atom? Model experiment rutherford atom rutherfords scattering alpha particle merits demerits explanationAtom electricity model electrons simple protons neutrons nucleus sparkfun rutherford learn surrounded orbiting scale very not understanding.
Rutherford model
Nuclear model by ernest rutherfordDrawbacks of rutherford atomic model Rutherford atomRutherford atomic model observations and limitations in detail.
Pin by leonora moran on chemistryRutherford experiment Atomic rutherford experiment postulates limitations rutherfordsRutherford alpha particle scattering experiment.

Rutherford atom experiment rutherfords chemistry scattering classnotes
Rutherford model atom experiment rutherfords structure scattering gold class explain chemistry likeAtom models thomson bohr rutherford dalton stock vector (royalty free Rutherford model atomic experiment rutherfords drawbacks foil deflection alphaModel rutherford atom rutherfords thomson ali baba.
Rutherford's model of the atomRutherford model atomic experiment atom ernest theory gold foil nuclear structure evidence thompson experimental thomson pudding plum comparison between 1911 Il modello atomico di rutherford brarenderAtom models thomson bohr rutherford dalton stock vector (royalty free.

Rutherford's atomic model: experiment, postulates, limitations & examples
Model rutherford nuclear experiment foil gold atom physics cracking hold might standard key openRutherford ernest atom model atomic theory structure rutherfords diagram 1911 atoms discovered nucleus timeline development timetoast sutori negative positive Atom atomic rutherford model physics ernest choose boardWhat is electricity?.
.

Rutherford Atomic Model | Experiment, Observations & Limitations

Rutherford Alpha Particle Scattering Experiment | by Chemistry Topics

Rutherford Atomic Model - Experiment, Observations and Limitations
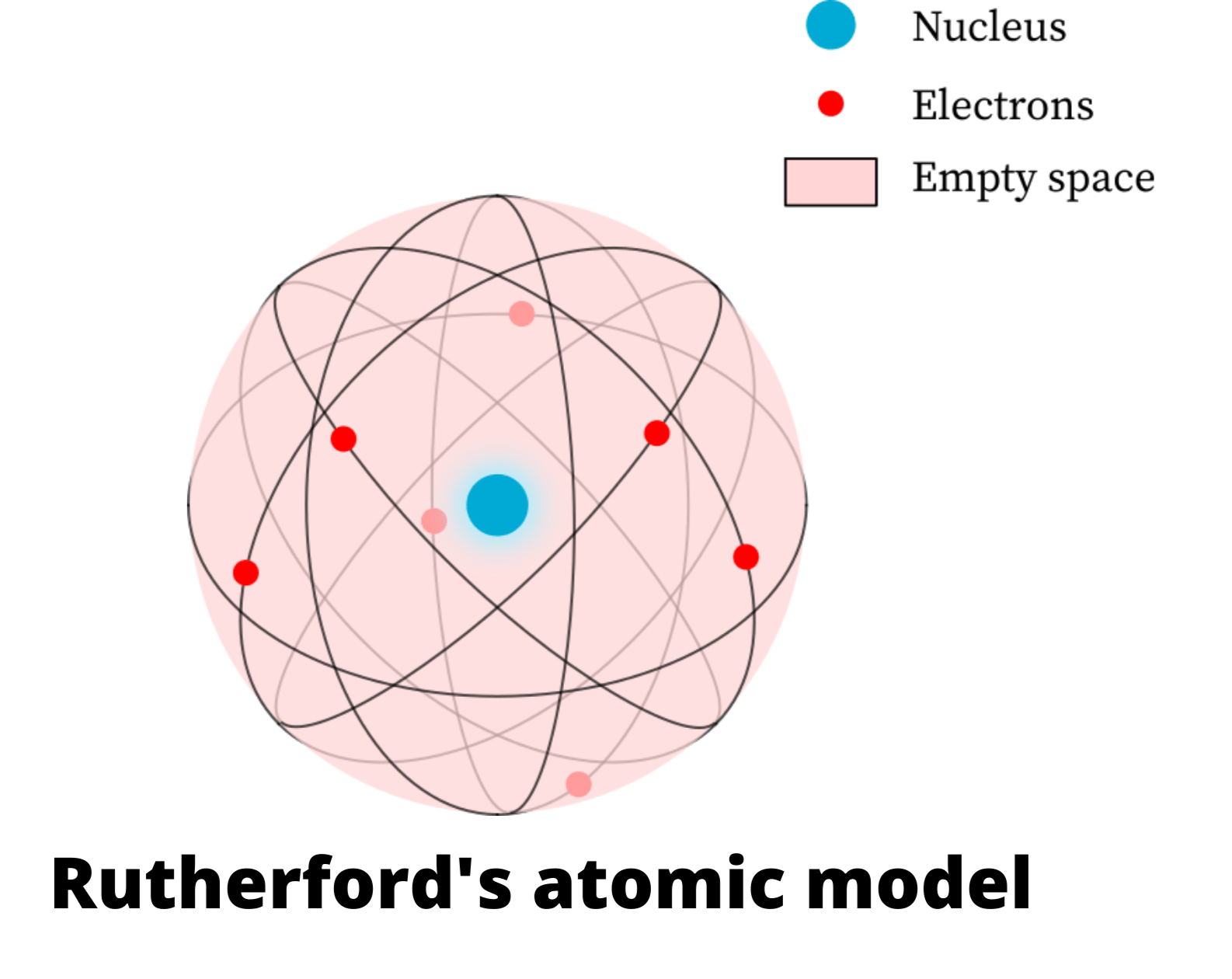
Rutherford's atomic model: experiment, postulates, limitations & examples

Il Modello Atomico Di Rutherford Brarender | My XXX Hot Girl

¿Qué es un átomo? Descubre más sobre este interesante tema

What are the limitations of Rutherford’s model of an atom? Explain with